Durable labelling solutions for medical devices, hospitals, practices, laboratories as well as care and rehabilitation facilities
Whether in hospitals or laboratories, with medical products or in the pharmaceutical industry: correct and clear labelling not only makes work in the medical environment easier, it can also save lives. Constantly increasing legal and regulatory requirements for medical products and drugs, ensuring that patients receive the best possible care at all times, has meant increasing cost pressure in hospitals and clinics.
Barcode and RFID solutions from inotec can help. You can count on our many years of experience and a wide range of labels specially adapted to the challenges of the healthcare sector. No matter what mechanical, thermal or chemical stresses need to be overcome, together we will find a customized solution for labelling your medical devices. From blood bag and sample labels to tamper evident security seals, we offer consulting, development and manufacturing as a complete package. We can also provide you with information on identification within the framework of the UDI (the EU's binding directive on the unique identification of devices).
THE ADVANTAGE OF WORKING WITH INOTEC
- Individually customized solutions for all the latest challenges (tracing of goods, product protection, unique numbering and tamper evident)
- Unique labelling for the implementation of applicable directives (e.g. UDI) ISEGA-certified, medically safe and diffusion-free adhesives
- Robust and durable labels for demanding applications without altering the product surface
- Product tests in our own Label Competence Centre
- Advice, all-round service and long-term support from our experts
UDI: new obligations for medical device labelling
The transition period is long over: starting in May 2021, all medical devices must bear a unique sequential product identifying number, starting with class III. This number will be affixed to the product and/or product packaging in digitally readable form (e.g. as a data matrix code). Class II and I will follow later. With inotec barcode and RFID solutions you can stay ahead of these requirements. From individual labels for unique identification to complete product labels, we can provide you with UDI-compliant solutions for all medical products. Individually adaptable to bespoke requirements and completely flexible, for printing yourself or pre-printed by us. Or grab your chance right now and turn the UDI requirements into a real competitive advantage: optimize your processes in procurement, logistics and production through clear product labelling, and future proof, for example with modern RFID smart label technology, and be one step ahead of the competition.
The right labels for inventory and medical products
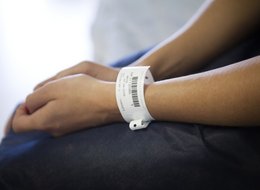
For permanent labelling and the full traceability this offers, for blood bags, specimen or samples, we can offer various solutions, each adapted to the strengths required in different application areas. Which label construction is chosen will therefore depend first and foremost on the use of the product to be identified. There is a difference between identification by laser engraving and identification by barcode and/or RFID label). Both are scratch and chemical resistant and can be used on a wide variety of materials, as well as on very small surfaces. We have compared the advantages and disadvantages of both methods.
Identification by means of laser engraving | Identification with digital labels |
|---|---|
Advantages
| Advantages
|
Disadvantages
| Disadvantages
|
Although the laser is particularly useful for coding products that require very intensive cleaning, such as surgical instruments, our labels are suitable for virtually all other medical-technical applications. Many of these instruments can, of course, also be identified with a laser, while in some cases it may also make sense to identify surgical instruments with RFID solutions. Nevertheless, inotec's labels offer many advantages: they are not only resistant to heat and cold, but also ISEGA-certified, medically safe and can even be produced just-in-time in a self-printing process. Use inotec’s labels on medical equipment is just one possible application as labels can also be of value in patient management, asset tracking, product protection or the management of consumables.
Well advised: Labelling solutions from inotec for hospitals, laboratories and medical practices
The method you choose for labelling and implementing the UDI Directive depends heavily on the devices and products to be labelled. That is why we cannot and do not want to make a general recommendation at this point. Both lasers and labels have their raison d'être and offer secure and efficient options for marking. Nevertheless, the extensive possibilities offered by barcodes and RFID systems today make labels extremely attractive, especially for the medical and pharmaceutical industries. With our label solutions, which are individually tailored to your circumstances and which we test extensively in advance in our in-house Label Competence Center, you not only implement applicable legal requirements, but also optimize your internal processes at the same time. Let our experienced experts advise you without obligation and invest in the safety of your products and the future of your company.